PVC (Polyvinyl chloride) Granules
First of all, what is Polyvinyl Chloride (PVC)? Polyvinyl Chloride (PVC) is one of the most commonly used thermoplastic polymers worldwide. It is naturally white and very brittle plastic. PVC has been around longer than most plastics ,first synthesized in 1872 produced by B.F. Goodrich Company in the 1920s. It is used most commonly in the construction industry and is also used for signs, healthcare applications, and fiber for clothing.
PVC is produced in two general forms: a rigid or unplasticized polymer (RPVC), and the second as a flexible plastic. . In its base form, PVC is characterized by its rigid yet brittle structure. While the plasticized version holds various uses across multiple industries, the rigid version of PVC also has its share of uses. Industries such as plumbing, sewage, and agriculture can utilize rigid PVC across many functions. Flexible PVC is commonly used in construction as insulation on electrical wires or in flooring for homes, hospitals, schools, and other areas where a sterile environment is a priority. In some cases, PVC can act as an effective replacement for rubber.
Some of PVC plastic’s most important characteristics include its relatively low price, its resistance to environmental degradation (as well as to chemicals and alkalis), high hardness, and outstanding tensile strength for plastic in the case of rigid PVC. It remains widely available, commonly used, and easily recyclable.

Applications of PVC
Basic Configurations Of PVC
- Flexible PVC, also known as PVC-P, has a density of 1.1-1.35 g/cm3 and is produced by adding compatible plasticizers to PVC, decreasing the material’s crystallinity. The addition of these plasticizers results in a more flexible and transparent plastic.
- Rigid PVC, also known as UPVC, PVC-U, or uPVC, has a density of 1.3-1.45 g/cm3 and is a stiff and economical plastic that offers high resistance to impact, water, weather, chemicals, and corrosive environments.
- Molecular Oriented PVC, also known as PVC-O, is formed by reorganizing the amorphous structure of PVC-U into a layered structure. This process produces a material with enhanced physical characteristics, such as increased stiffness, fatigue resistance, and lightness.
- Chlorinated Polyvinyl Chloride (CPVC) is produced by chlorinating PVC resin, resulting in a material with a high chlorine content that offers high durability, chemical stability, and flame retardancy. This material can withstand a wider range of temperatures.
- Finally, Modified PVC, also known as PVC-M, is an alloy formed by adding modifying agents to the material, which results in enhanced toughness and impact properties.
How Is PVC Made?
Vinyl chloride monomer (VCM) is created through the chlorination of ethylene followed by the pyrolysis of the resulting ethylene dichloride (EDC) in a cracking unit.
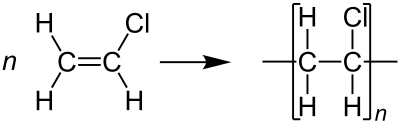
The polymerization of vinyl chloride monomer (VCM) produces PVC, which has a glass transition temperature of 70-80°C.
There are two standard methods used to produce PVC commercially:
- Suspension PVC (S-PVC)
- Bulk or Emulsion (E-PVC)
Suspension PVC (S-PVC)
In the Suspension PVC (S-PVC) process, the monomer, the polymerization initiator, and other additives are introduced into a pressure-tight reactor.
The reaction vessel’s contents are continuously mixed to maintain suspension and ensure uniform particle size of PVC resin.
Typically, suspension polymerized PVC has a mean particle size of 100-150 µm with a 50-250 µm range.
S-PVC grades are formulated to meet extensive requirements, such as high plasticizer absorption for flexible products, high bulk density, and good powder flow for rigid extrusion.
Suspension polymerization accounts for 80% of PVC production worldwide.
Bulk or Emulsion (E-PVC)
In the Bulk or Emulsion (E-PVC) process, surfactants (soaps) disperse the vinyl chloride monomer in water.
The monomer is trapped inside soap micelles and is protected by the soap, allowing polymerization to occur using water-soluble initiators.
The primary particles are solid, smooth-surfaced spheres that cluster into irregularly shaped aggregates, with a mean particle size of 40-50 µm and a range of 0.1-100 µm.
E-PVC resins are used in various specialty applications such as coating, dipping, or spreading.
PVC polymers are linear and robust.
The monomers are arranged predominantly in a head-to-tail configuration, with chlorides alternating on carbon centers.
PVC exhibits a primarily atactic structure, resulting in a random distribution of chloride centers.
Some degree of syndiotactic in the chain contributes a small percentage of crystallinity, significantly impacting the material’s properties, such as ,melting point
With chlorine accounting for approximately 57% of the mass, PVC’s properties differ significantly from those of structurally related materials, such as polyethylene.
Additionally, PVC has a higher density than these associated plastics
References
Introduction https://www.nirmalvasundhara.com/blog/what-is-polyvinyl-chloride
